Blog Standard

Advancing Biomarker Discovery Through Circulating Tumor Cells Analysis
Biomarker discovery is crucial in understanding cancer progression, identifying tumor-specific markers, and evaluating drug target expression for personalized treatment. One of the most promising areas of research in oncology is the study of circulating tumor cells (CTCs), which provide insights into tumor heterogeneity and response to therapy. These cells, shed from primary or metastatic tumors into the bloodstream, offer a minimally invasive method for cancer monitoring and treatment decision-making.
RareCyte’s Developer Kit technology plays a significant role in advancing CTC enumeration and single cell analysis, allowing researchers to examine molecular characteristics and phenotypic heterogeneity at an unprecedented level. This study explores the application of RareCyte Developer technology in breast cancer patients, lung cancers, and other malignancies through circulating tumor cells blood tests.

Methods
To evaluate the effectiveness of circulating tumor cells tests, blood samples were spiked with both positive and negative model circulating tumor cells (mCTC-positive and mCTC-negative). These samples were processed using the AccuCyte® Sample Preparation System, ensuring high recovery and purity of CTCs.
The staining process involved the RarePlex® CTC Panel Kit, which employs a three-channel detection method:
- Nuclear dye for cellular identification
- Anti-CD45 antibody to exclude white blood cells
- Cocktailed antibodies to detect epithelial mesenchymal transition (EMT) markers such as cytokeratin (CK) and epithelial cell adhesion molecule (EpCAM)
Furthermore, RareCyte’s Developer Kit technology was utilized to investigate additional tumor-specific markers including HER2, ER, PR, EGFR, Ki67, AR, ARv7, and PSMA. The stained slides were imaged using CyteFinder® Instrument, and CTC identification was performed through machine learning-based algorithms with manual confirmation via CyteHub® software.
Results
The study successfully demonstrated the efficiency of RareCyte Developer Kits in detecting a wide range of biomarkers in both control and clinical samples. The results highlighted the following key findings:

Fig1: Clone specificity evaluation by mCTC-positive and mCTC-negative cell lines.
- luorescence intensity cut-offs were statistically defined to distinguish between positive and negative marker expression.
- Breast cancer patients and lung cancers samples exhibited staining patterns consistent with expected biomarker localization.
- The ability to retrieve and analyze single cells from a heterogeneous tumor population enabled a more refined understanding of tumor heterogeneity and phenotypic heterogeneity.
- Custom assays developed with RareCyte Developer technology provided accurate and reproducible biomarker detection.
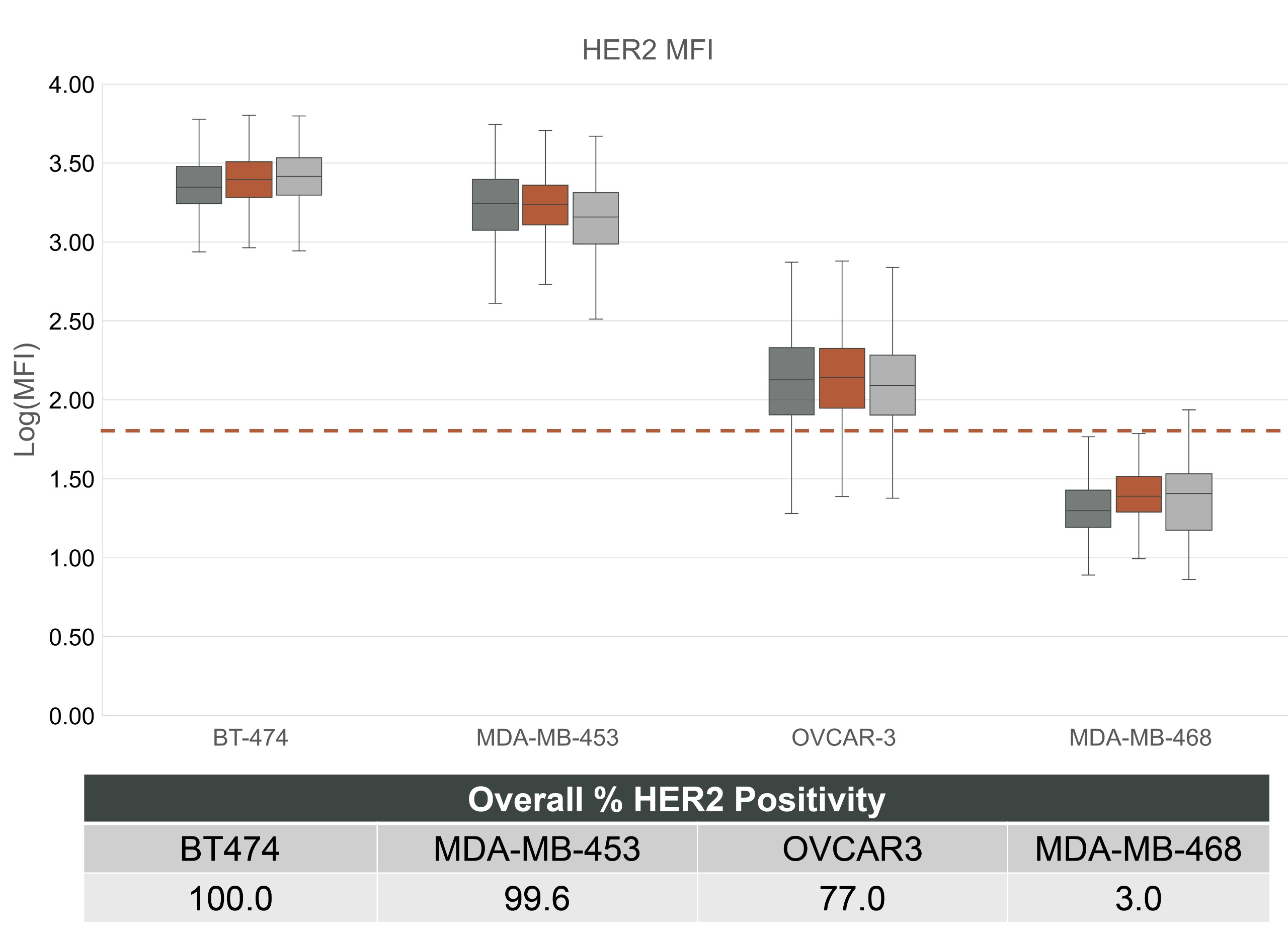
Fig 2: MFI comparison for mCTCs with a range of HER2 expression.
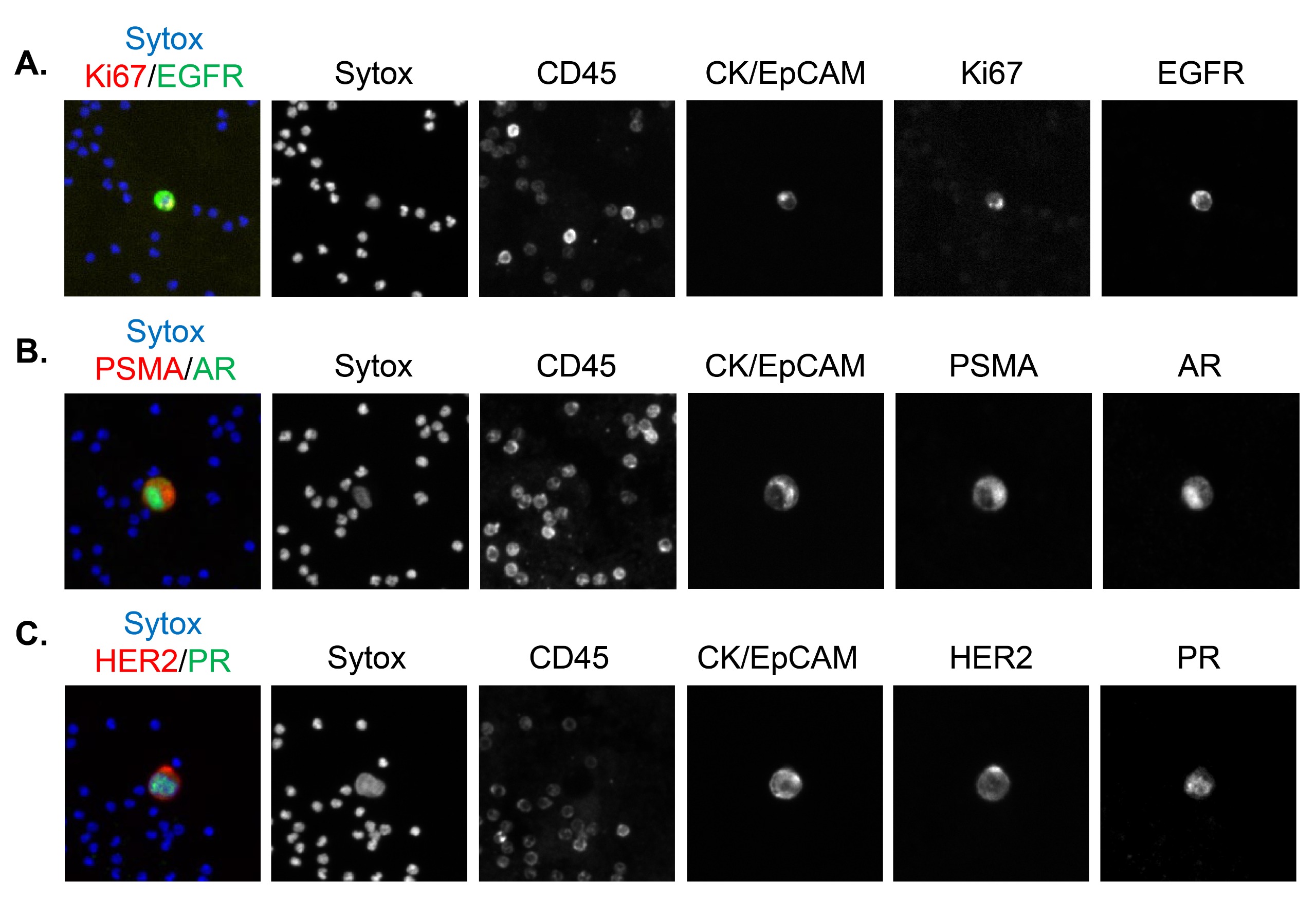
Fig 3: mCTCs staining with expected subcellular localization using the base epithelial CTC detection assay and two additional biomarkers.
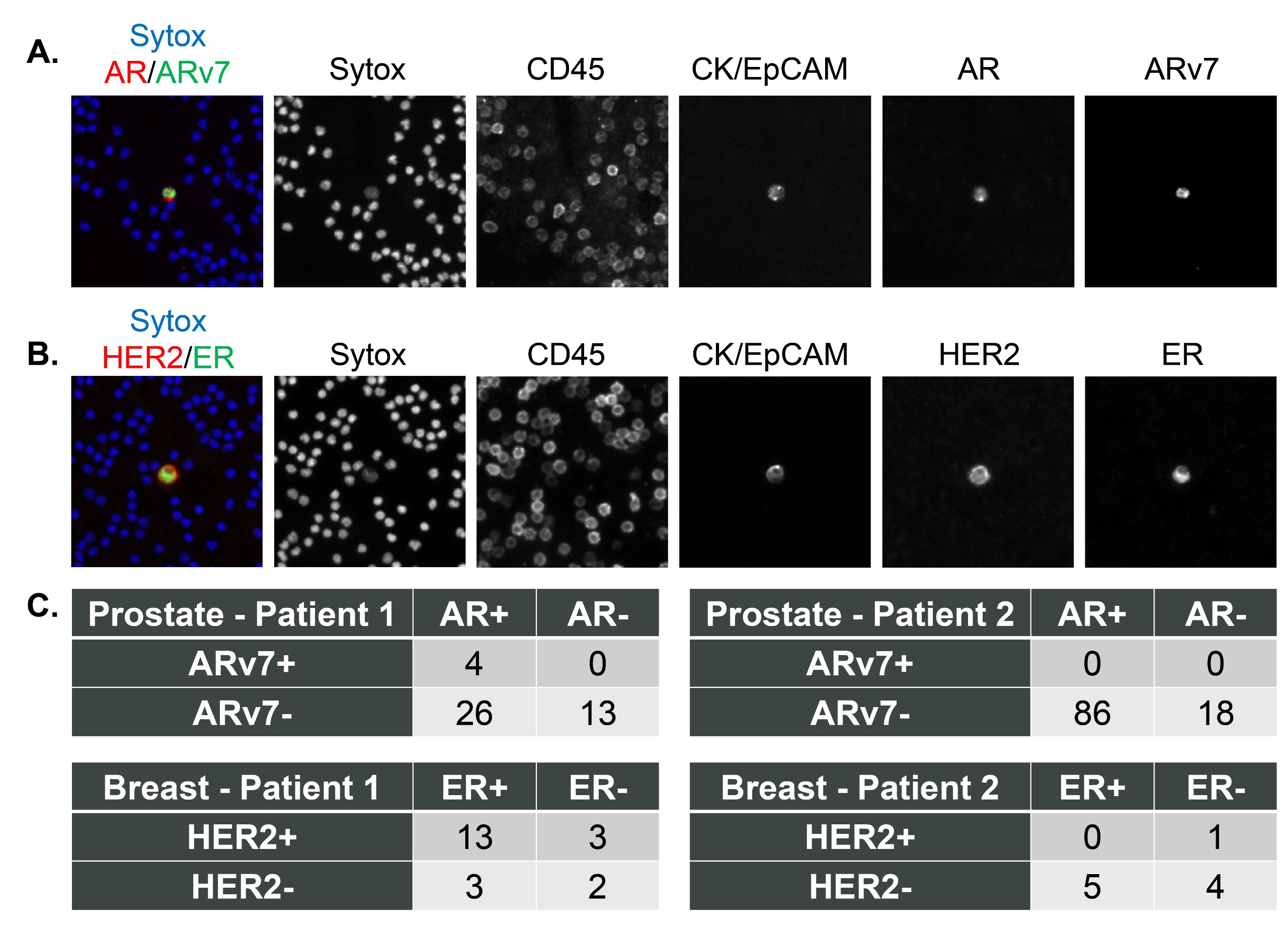
Fig 4: Clinical samples stained using the base epithelial CTC detection assay and two additional biomarkers.
Conclusion
The integration of RareCyte Developer technology with CTC enumeration and single-cell retrieval has significantly enhanced the ability to conduct molecular analysis on circulating tumor cells. This advancement has profound implications for cancer research, particularly in assessing drug target expression, monitoring response to therapy, and improving early detection strategies.
With the growing awareness of breast cancer and its impact on patients worldwide, circulating tumor cells blood tests provide a valuable tool for personalized medicine, allowing for targeted treatment approaches based on individual tumor cells characteristics. As research progresses, the continued development of thermoanalytical methods and customized biomarker assays will further enhance our ability to combat cancer with precision and efficiency.
Inkarp Instruments is a reliable distributor and service provider of Rarecyte products in India, delivering innovative scientific solutions. Dedicated to quality and dependability, we empower researchers across the country with premium products and outstanding support.
References
1. Campton et al., (2019).Custom biomarker investigation of circulating tumor cells using RarePlex®Developer Kits. Univ of Washington, RareCyte, Seattle.

