Blog Standard

Accurate And Precise Spike-In Method For Ctc Validation Studies With Cellenone®
The analysis of circulating tumor cells (CTCs) from a simple blood draw holds significant promise for cancer diagnostics and monitoring treatment progress. Given the extreme rarity of CTCs—often occurring at a rate of just 1 CTC per 10 billion red blood cells—advanced technologies like RareCyte's AccuCyte®- CyteFinder® system are essential. This system offers exceptional sensitivity and specificity while delivering a consistent and comprehensive workflow.
Accurate, precise, and reproducible CTC detection is crucial for diagnostics and clinical trials, necessitating thorough assay validation. To validate enumeration accuracy, it is essential to create surrogate blood samples containing known quantities of model CTCs (mCTCs) at levels close to the assay's detection limit. Achieving this requires precise and reproducible spike-in samples, a task that cannot be reliably accomplished using serial dilution or flow cytometry methods.
CellenONE®: Transforming Single-Cell Isolation and Dispensing
Developed by Cellenion, the cellenONE® instrument is an advanced single-cell isolation and dispensing technology that uses automated image recognition to ensure each droplet contains precisely one cell. While the primary applications of cellenONE are in single-cell analyses and cell line development, Cellenion and RareCyte have collaborated to extend its use to a novel domain: circulating tumor cell (CTC) assay validation. This collaboration demonstrates how RareCyte employs cellenONE to generate critical samples for CTC assay validation, establishing it as the preferred system for mCTC sample preparation.
Methods
A customized sample deck was engineered for cellenONE to facilitate precise cell deposition on both microscope slides and RareCyte’s AccuCyte Blood Collection Tubes (BCTs) containing 7.5 ml of whole blood (Figure 1).

Figure 1. 5-step CTC enumeration validation workflow. Dispense cells with cellenONE onto slides and into BCT; process BCT to slides with AccuCyte system; stain slides with RarePlex® Enumeration Panel Kit; image and analyze with CyteFinder Instrument to count CTCs.
The cellenONE was programmedtodispense:
- ● 100cells per slide
- ● 5–8cells per BCT
Blood samples were subsequently processed onto slides using the AccuCyte system. After processing, mCTCs (model CTCs) on all slides were counted using RareCyte’s CTCenumeration assay.
Results and Discussion
The cellenONE instrument enabled the deposition of known numbers of cells with exceptional accuracy and precision, supporting standardized sample preparation for assay validation studies.
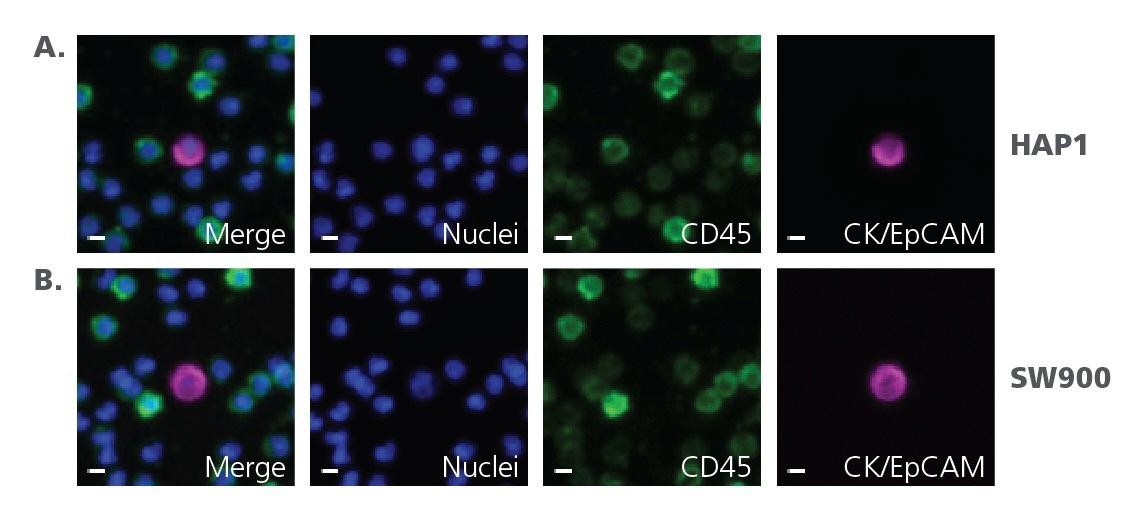
Figure 2. mCTC images from cellenONE spike-in assay. CTCs are identified by the presence of a nucleus, localization of Cytokeratin (CK) to the cytoplasm or Epithelial Cell Adhesion Molecule (EpCAM) to the cell membrane, and absence of CD45 localization.
Figure A. HAP-1 mCTC spike-in assay.
Figure B. SW900 mCTC spike-in assay. Scale bar represents 5 μm.

Figure 3. Image of 100 mCTCs printed to a slide.
Single-Cell Dispensing Accuracy: The system’s accuracy was confirmed by directly printing single cells onto slides, as shown in Table 1.
Spike-In Studies: The cellenONE was then employed to spike limiting numbers of mCTCsintoblood tubes. When processed with the RareCyte CTC assay, these samples demonstrated remarkable recovery rates, as detailed in Table 2.
The cellenONE method provided a reproducible solution for dispensing clinically relevant quantities of cells, which is critical for robust assay validation.
| Replicate | Print # | mCTC count |
| 1 | 100 | 100 |
| 2 | 100 | 100 |
| 3 | 100 | 100 |
| 4 | 100 | 100 |
| 5 | 100 | 99 |
| 6 | 100 | 99 |
| 7 | 100 | 100 |
| 8 | 100 | 99 |
| Total | 800 | 797 |
| Recovery | 0.99 |
Table 1: mCTC count for slides printed with 100 mCTCs.
| Replicate | Spike-in # | mCTC count |
| 1 | 8 | 8 |
| 2 | 5 | 5 |
| 3 | 5 | 5 |
| 4 | 5 | 4 |
| 5 | 5 | 5 |
| 6 | 5 | 4 |
| 7 | 5 | 5 |
| 8 | 5 | 5 |
| 9 | 6 | 6 |
| Total | 49 | 47 |
| Recovery | 0.99 |
Table 2: mCTC count from deposition of 5-8 cells per BCT
Conclusion
In clinical diagnostics, accuracy, precision, and reproducibility are non-negotiable. RareCyte, known for its high-performance CTC assays, ensures rigorous validation processes to meet these demands.
For accuracy validation studies, RareCyte required a system capable of reliably dispensing single mCTCs with unparalleled precision. Existing methods like flow cytometry and serial dilution assays fell short in achieving high accuracy for single-digit cell deposition.
The cellenONE system fulfilled these requirements, making it an integral component of RareCyte’s validation process for every commercially developed CTC assay. By leveraging cellenONE technology, RareCyte has elevated its assay validation standards, ensuring consistency and reliability in clinical diagnostics.
References - RareCyte

