Blog Standard
Exploring Cell-Based Liquid Biopsy: Beyond Circulating Tumour Cell Enumeration with the RareCyte Platform
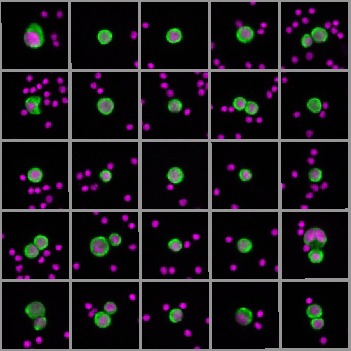
In recent years, liquid biopsy has revolutionised cancer diagnostics. Unlike traditional tissue biopsies, which require invasive procedures, liquid biopsy allows for the non-invasive collection of blood samples to analyse circulating tumour DNA (ctDNA) and circulating tumour cells (CTCs). However, while ctDNA has been widely adopted for mutational analysis, it lacks the ability to assess cancer phenotypes, such as protein biomarker expression, which can be crucial in guiding treatment decisions. This is where CTCs come into play. CTCs are intact cancer cells that have detached from a tumour and entered the bloodstream, potentially leading to distant metastases. Their presence in the blood is not only a marker of disease progression but also of resistance to cancer therapies.
Liquid Biopsy
The RareCyte Liquid Biopsy Platform
The RareCyte platform offers a comprehensive workflow for CTC analysis that includes:
- Sampleprocessing: Using the AccuCyte® system to collect blood and separate nucleated cells.
- Immunofluorescence staining: Using RarePlex® kits to stain specific protein markers.
- Imaging:CyteFinder, an automated scanning microscope, captures high-resolution images.
- Nucleic acid sequencing: The CytePicker® device allows for precise retrieval of CTCs for genetic sequencing.
This workflow enables both the enumeration of CTCs and in-depth analysis of protein biomarkers and genomic information, expanding the potential of liquid biopsy beyond traditional methods.
Protein Biomarker Analysis
One of the most exciting aspects of the RareCyte platform is its ability to investigate protein biomarkers on CTCs. These biomarkers can provide critical insights into tumour characteristics, drug targets, and potential resistance mechanisms. For instance, the platform has validated assays for biomarkers like the androgen receptor (AR) in prostate cancer and HER2 and ER in breast cancer. This allows clinicians to track the presence of different cancer subtypes over time, offering a dynamic view of the tumour’s evolution.
The RareCyte platform can also support companion diagnostic development. By analysing drug targets directly on CTCs, researchers can better understand how patients might respond to therapies and develop non-invasive diagnostic tests.
Nucleic Acid Sequencing Applications
CTCs offer a distinct advantage over ctDNA for genomic analysis. Unlike ctDNA, which represents fragmented genetic material, CTCs contain entire cancer genomes. This allows for:
- Targeted mutation analysis: RareCyte has been used to detect actionable mutations in CTCs that were missed in plasma ctDNA, offering a more sensitive approach to mutation detection.
- Wholegenome/exome sequencing: By sequencing the entire genome of CTCs, researchers can track the genomic evolution of cancer over time.
- Resistant clone detection: CTCs can be isolated and sequenced during therapy to identify mutations in resistant cell populations, which is critical for planning targeted treatments.
Clinical Applications and Breakthroughs
In clinical settings, the RareCyte platform has shown remarkable results. In a study involving gastrointestinal cancer patients, CTC enumeration and biomarker analysis provided real-time insights into treatment responses. For instance, CTCs were characterised using dual-biomarker assays to assess proliferation markers like EGFR and Ki-67, as well as immune checkpoint markers such as HER2 and PD-L1. This type of analysis allows for more personalised and effective treatment strategies.
In another example, CTCs from a colorectal cancer patient were sequenced using a targeted gene panel, identifying an APC mutation in some CTCs. This kind of detailed genomic analysis can help clinicians tailor therapies to the unique genetic makeup of each patient’s cancer.
Conclusion:
The RareCyte platform is pushing the boundaries of liquid biopsy by enabling not just the enumeration of CTCs but also detailed phenotypic and genomic analysis. By combining CTC biomarker analysis with ctDNA sequencing, liquid biopsy can offer a more comprehensive view of cancer, helping clinicians make more informed decisions. As we move toward more personalised medicine, technologies like RareCyte are essential in harnessing the full potential of liquid biopsy to improve cancer diagnosis, treatment, and patient outcomes.
References:
Kaldjian, E. P.; Ramirez, A. B.; Costandy, L.; Ericson, N. G.; Malkawi, W. I.; George, T. C.; Pashtoon Murtaza Kasi. Beyond Circulating Tumor Cell Enumeration: Cell-Based Liquid Biopsy to Assess Protein Biomarkers and Cancer Genomics Using the RareCyte® Platform. Frontiers in Pharmacology 2022, 13. DOI: 10.3389/fphar.2022.835727.

