Blog Standard

A New Biomarker for NSCLC Immunotherapy: Functional PD-1/PD-L1 Engagement
Immunotherapies for non–small-cell lung carcinoma (NSCLC) have made significant strides in recent years. However, despite considerable success in certain subsets of patients, a substantial proportion of patients do not benefit from these therapies.
But, a groundbreaking case study by Hawk Biosystems featured in the Journal of Clinical Oncology sheds new light on immunotherapy in non-small cell lung carcinoma (NSCLC). Titled “Functional engagement of the PD-1/PD-L1 complex but not PD-L1 expression is highly predictive of patient response to immunotherapy in NSCLC,” the findings of the case study suggest that by improving patient selection methods, response rates to immune checkpoint inhibition (ICI) therapies in non-small cell lung carcinoma (NSCLC) could increase by up to 280%.
Understanding Immune Checkpoints
Immune checkpoints are receptor-ligand pairs that naturally regulate the immune system to prevent excessive activation, which could lead to autoimmunity. Key immune checkpoints include:
- ProgrammedDeath Receptor-1/Programmed Death Ligand-1 (PD-1/PD-L1)
- Cytotoxic T-Lymphocyte Associated Protein-4/CD80 (CTLA-4/CD80)
- T-Cell Immunoreceptor with Ig and ITIM domains/CD155 (TIGIT/CD155)
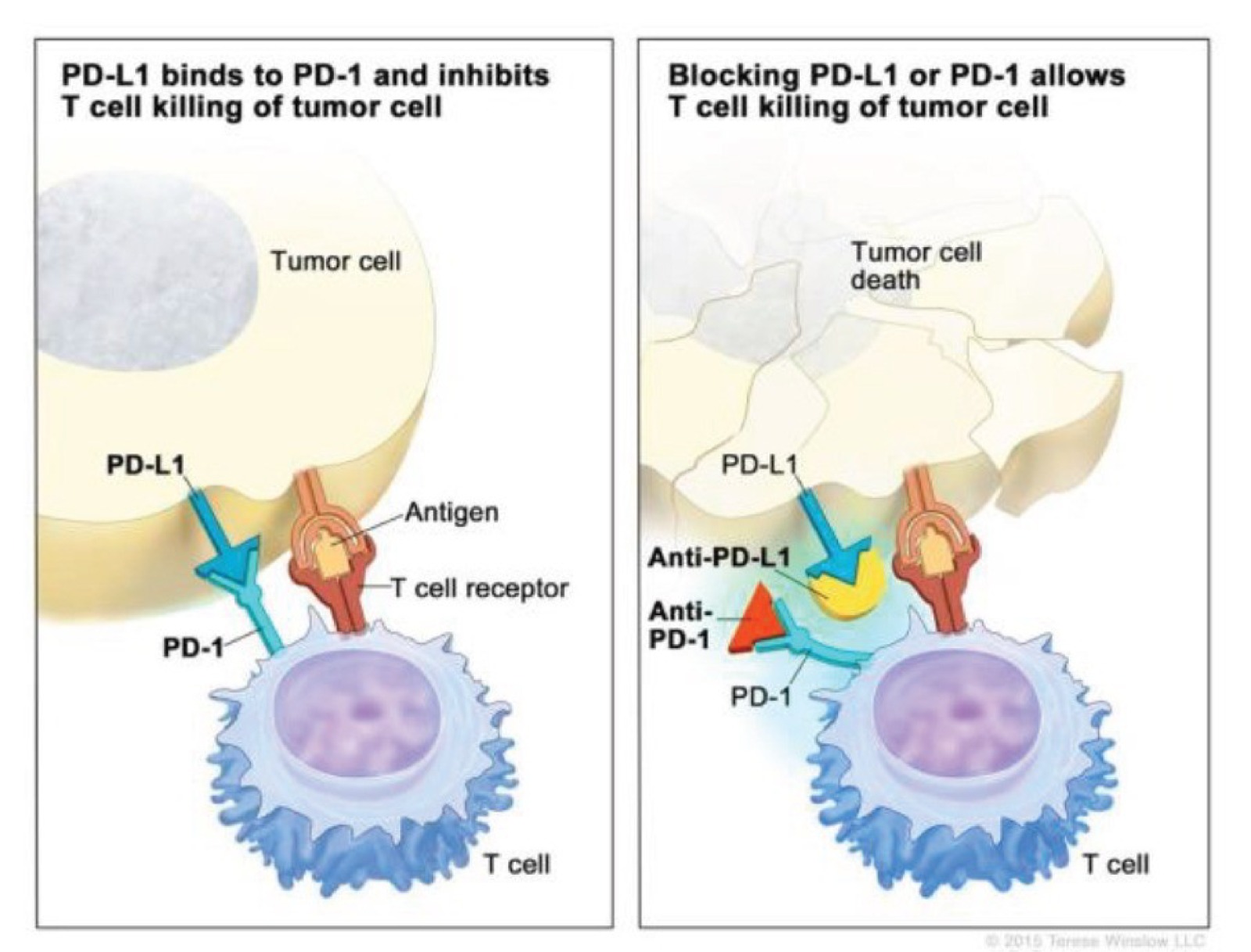
Figure 1: PD-1/PD-L1 is an immune checkpoint designed to switch off the immune system to prevent auto-immune disease. However, some cancers upregulate the ligand to evade the immune system, which is is inherently programmed to destroy neoplastic cells. Drugs blocking these interactions between the immune system and cancer cells can allow the immune system to find and clear cancer cells from the body.
For instance, the PD-1/PD-L1 checkpoint inhibits immune activity to prevent tissue damage. However, many cancers, including NSCLC, exploit this mechanism by overexpressing PD-L1, effectively evading immune destruction. Therapeutic monoclonal antibodies targeting these interactions have shown promise in reactivating the immune response against tumors. Yet, challenges persist as many patients either do not respond or develop resistance to these therapies due to suboptimal patient selection methods.
Current Methods for Patient Selection in ICI Therapy
Presently, NSCLC patients are selected for ICI therapies based on the expression levels of PD-L1 detected using immunohistochemistry (IHC). However, this approach has notable limitations:
- Non-Specificity of Antibodies: IHC antibodies may bind to unintended epitopes, leading to inaccurate PD-L1 quantification.
- Subjectivity in Readouts: Visual assessment and low-dynamic range scoring systems create inconsistencies across laboratories.
- Limited Predictive Value: High PD-L1 expression does not necessarily correlate with its interaction with PD-1.
As a result, patients are often misclassified:
- Somewithhigh PD-L1 expression but minimal PD-1/PD-L1 interaction receive ineffective treatments.
- Others with low PD-L1 expression but significant PD-1/PD-L1 interaction are excluded from therapies that could benefit them.
QF-Pro®: Revolutionizing Patient Stratification for ICI Therapies
QF-Pro® addresses these challenges by enabling precise quantification of protein-protein interactions (PPIs) and post-translational modifications (PTMs) using an innovative application of Förster Resonance Energy Transfer (FRET). This technology achieves unprecedented sensitivity and dynamic range, allowing for robust analysis even in pathology samples.
In the case study, PD-1 and PD-L1 were labeled with donor and acceptor chromophores, respectively. FRET signals, indicative of receptor-ligand interactions, were measured to identify patients most likely to benefit from ICI therapies. Unlike PD-L1 expression, these interactions strongly correlated with patient outcomes.
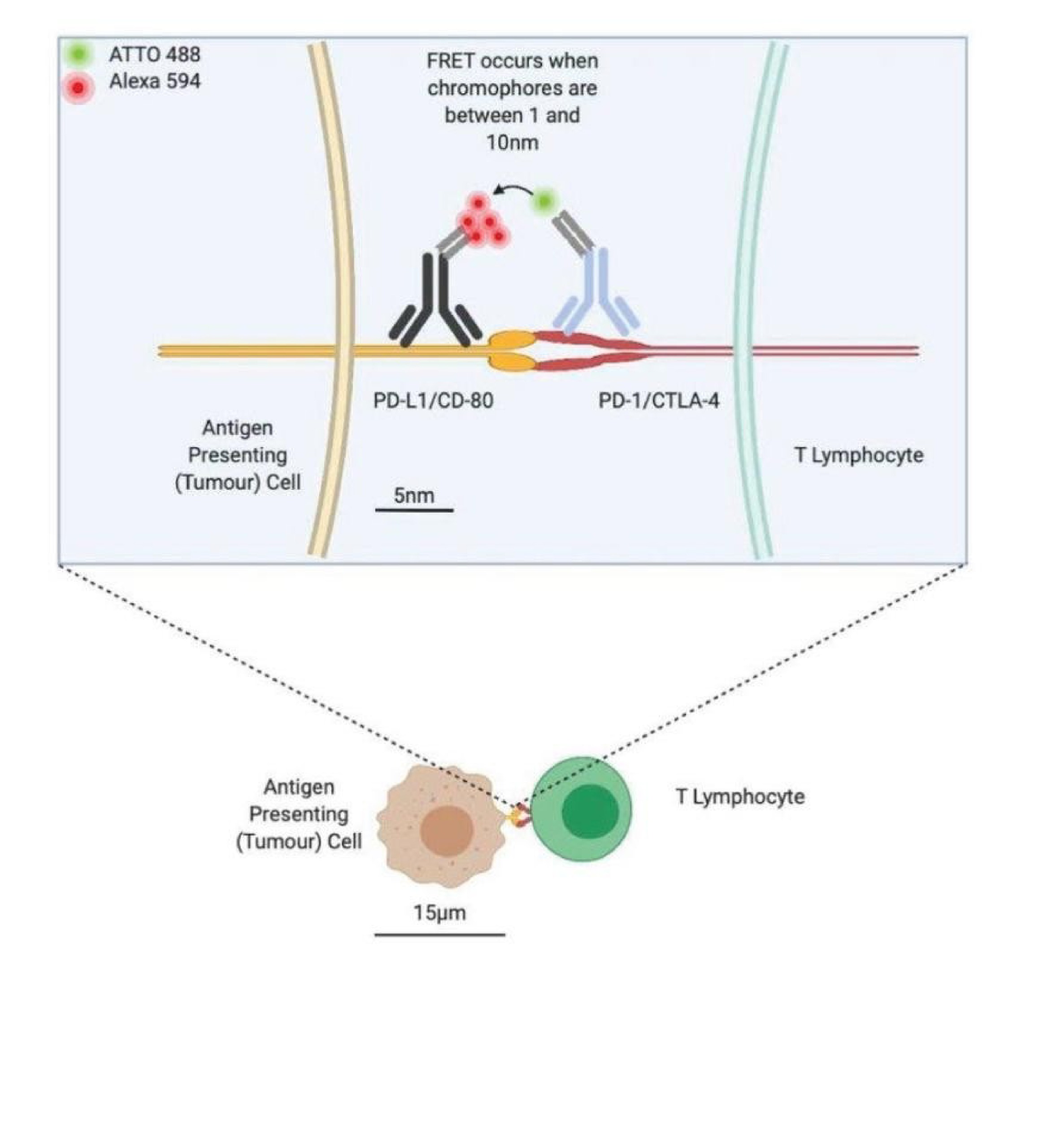
Figure 2: QF-Pro® detects FRET between the receptor (PD-1) and ligand (PD-L1) when they are interacting within 1-10nm.
Key Findings
A blinded, multi-site analysis of 188 NSCLC patients treated with ICIs revealed the following:
- LackofCorrelation: PD-L1 expression did not align with PD-1/PD-L1 interaction.
- Superior Predictive Value: Patients with high PD-1/PD-L1 interactions demonstrated significantly better responses and overall survival (OS) compared to those stratified by PD-L1 expression alone.
This method improved response rates to ICI therapies by up to 280%, addressing both patient under-treatment and over-treatment issues.
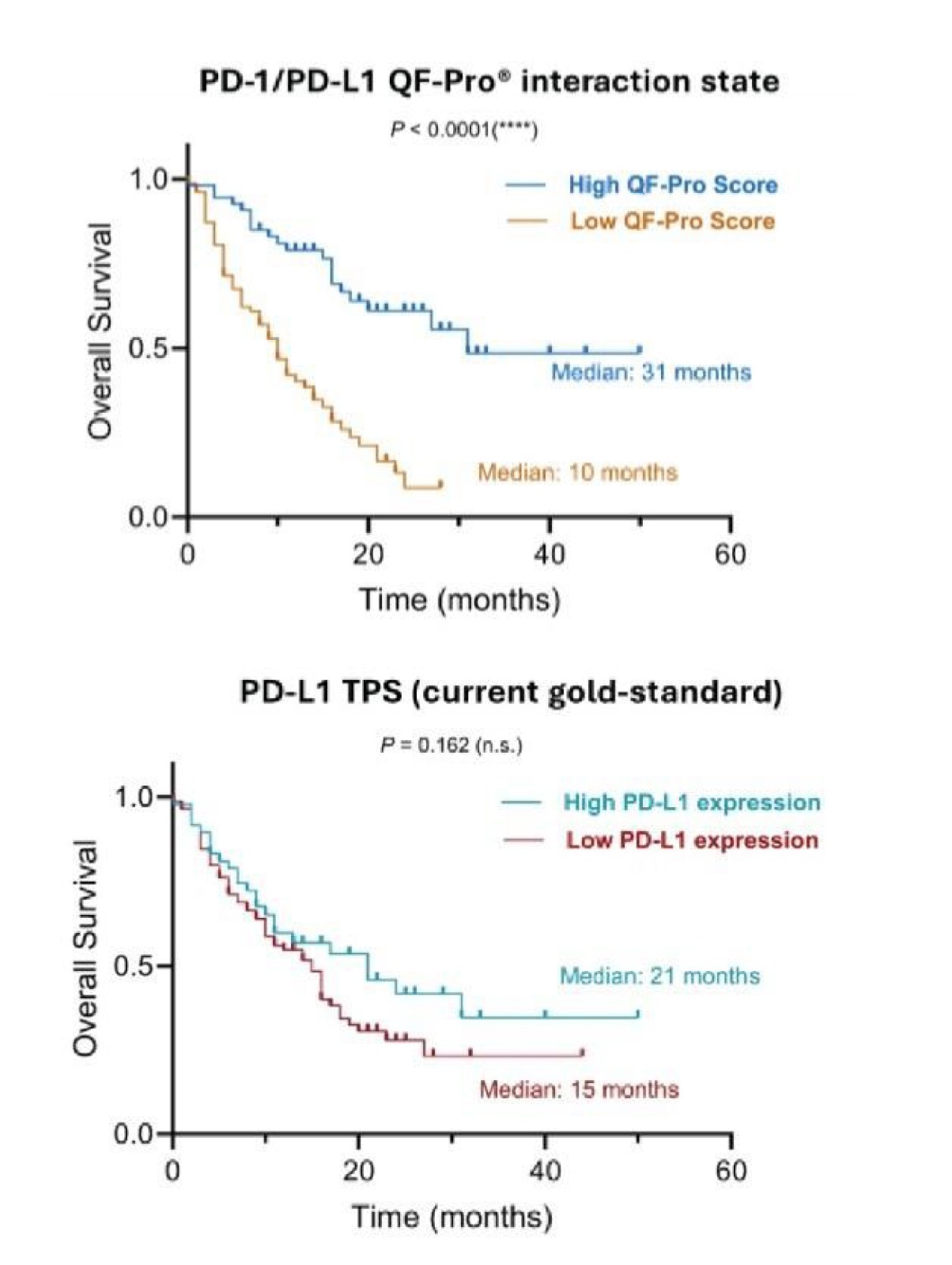
Figure 3: High PD-1/PD-L1 interaction state, quantify with QF-Pro® better correlates with enhanced OS in response to immunotherapy than PD-L1 expression. Patients were stratified into two groups: those with high interaction states and those with low interaction states.
Implications for Precision Medicine
The QF-Pro® platform represents a paradigm shift in personalized medicine, particularly in NSCLC. By identifying functional biomarkers like PD-1/PD-L1 interaction, it ensures accurate patient stratification, reducing unnecessary treatments and optimizing therapeutic efficacy.
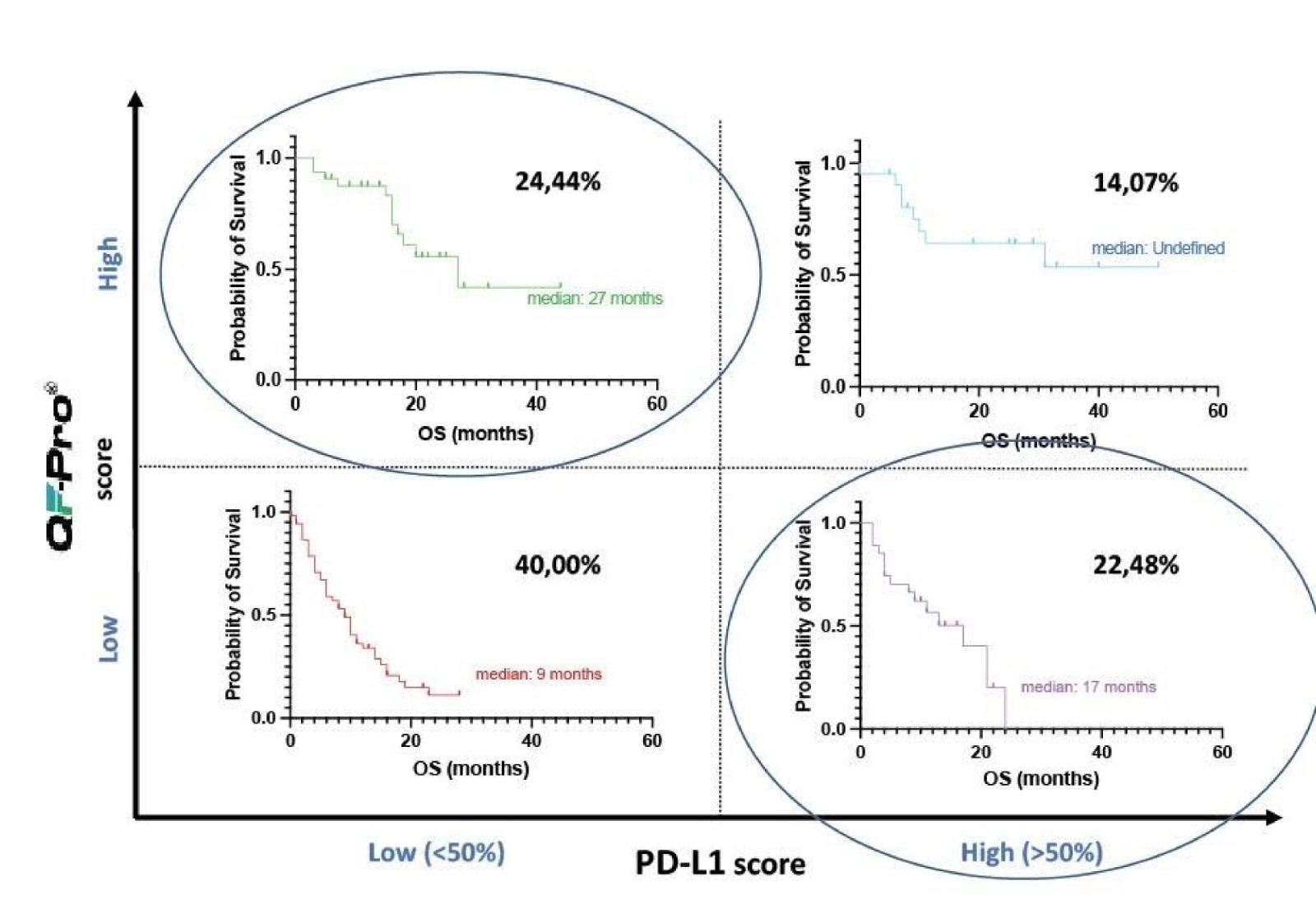
Figure 4: QF-Pro® identifies that 24.44% of NSCLC patients have significant PD-1/PD-L1 interaction state despite showing low PD-L1 scores. These NSCLC patients are currently missed from correct treatment groups.
Future Perspectives
The potential of QF-Pro® extends beyond NSCLC. Its application in other immune-oncology domains and fundamental research holds immense promise. HAWK Biosystems seeks collaborations to expand its deployment across larger cohorts and new clinical settings. Researchers and clinicians working on NSCLC, ICI therapies, or other pathological areas are encouraged to explore how QF-Pro® can advance their work.
Conclusion
The adoption of QF-Pro® has marked a monumental advancement in the field of personalized medicine. By replacing traditional PD-L1 expression-based stratification with PD-1/PD-L1 interaction analysis, this platform has the potential to significantly enhance patient outcomes in NSCLC and beyond. With its proven ability to accurately stratify patients and improve responserates, QF-Pro® represents a critical tool in the global agenda for precision medicine. HAWK Biosystems invites stakeholders to join in leveraging this technology to maximize its impact on patient care worldwide.
Reference
1. Aldarouish M, Wang C: Trends and advances in tumor immunology and lung cancer immunotherapy. J Exp Clin Cancer Res 35:157, 2016
2. Man J, Millican J, Mulvey A, et al: Response rate and survival at key timepoints with PD-1 blockade vs chemotherapy in PD-L1 subgroups: Meta-analysis of metastatic NSCLC trials. JNCI Cancer Spectr 5:pkab012, 2021
3. Qin S, Xu L, Yi M, et al: Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol Cancer 18155:155, 2019
4. Gridelli C, Ardizzoni A, Barberis M, et al: Predictive biomarkers of immunotherapy for non-small cell lung cancer: Results from an experts panel meeting of the Italian association of thoracic oncology. Transl Lung Cancer Res 6:373-386, 2017
5. Savic Prince S, Bubendorf L: Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Arch 474:475-484, 2019
6. Niu M, Yi M, Li N, et al: Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp Hematol Oncol 10:18, 2021
7. Veeriah S, Leboucher P, de Naurois J, et al: High-throughput time-resolved FRET reveals Akt/PKB activation as a poor prognostic marker in breast cancer. Cancer Res 74:4983-4995, 2014
8. Miles J, Applebee CJ, Leboucher P, et al: Time resolved amplified FRET identifies protein kinase B activation state as a marker for poor prognosis in clear cell renal cell carcinoma. BBA Clin 8:97-102, 2017
9. Sanchez-Magraner L, Miles J, Baker CL, et al: High PD-1/PD-L1 checkpoint interaction infers tumor selection and therapeutic sensitivity to anti-PD-1/PD-L1 treatment. Cancer Res 80:4244-4257, 2020
10. Sanchez-Magraner L, de la Fuente M, Evans C, et al: Quanti fication of PD-1/PD-L1 interaction between membranes from PBMCs and melanomasamplesusing cell membrane microarray and time-resolved forster resonance energy transfer. Analytica 2:156-170, 2021
11. Budczies J, Klauschen F, Sinn BV, et al: Cutoff finder: A comprehensive and straightforward webapplication enabling rapid biomarker cutoff optimization. PLoS One7:e51862, 2012

